
- Massive Range
- FREE UK Delivery
- Rapid Dispatch
- Massive Range
- FREE UK Delivery
- Rapid Dispatch
- Massive Range
- FREE UK Delivery
- Rapid Dispatch
Menu
Home » How Metals React: An Introduction to Metal Reactivity

We live in a world where metals play an important role. Whether it’s the coins in our pockets or the bridges and skyscrapers that fill our cities, metals are integral to our daily lives. But what makes metals so special, especially in terms of their reactivity? In this article, we explore the fascinating world of metal reactivity and examine how different metals react with various elements and compounds.
Metal reactivity refers to the ability of metals to engage in chemical reactions with other substances. The level of reactivity varies among metals, with some, like sodium, being highly reactive, while others, such as gold, are relatively inert. This reactivity is governed by the ease with which metals lose electrons and form positive ions or cations.
Electrons play a pivotal role in determining metal reactivity. Metals typically have loose electrons in their outer shell, which they can easily donate during reactions. The easier it is for a metal to lose its electrons and form cations, the more reactive it is.
Chemists have classified metals according to their reactivity into a series known as the reactivity series. This is a list that places the most reactive metals at the top and the least reactive ones at the bottom.
At the top of the list, metals like potassium and sodium react vigorously with water and oxygen, often resulting in explosions when exposed to these substances. On the other hand, gold, at the bottom of the list, is inert and does not corrode or tarnish over time, making it valuable for jewelry and monetary purposes.

To further understand metal reactivity, let’s delve into some common reactions that metals undergo:
Reaction with Oxygen (Oxidation): When metals react with oxygen, they form metal oxides. For instance, when magnesium burns in air, it forms magnesium oxide:
2Mg+O2→2MgO
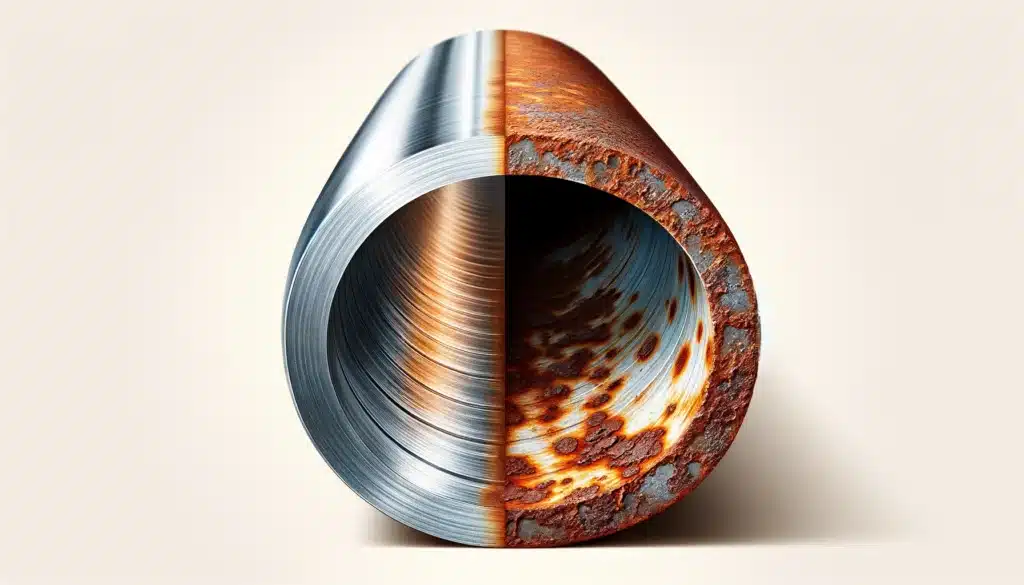
This is why metals like iron rust when exposed to moist air – they react with oxygen and water to form iron oxide.
Reaction with Water: Not all metals react with water, but those that do form metal hydroxides and hydrogen gas. Sodium reacts violently with cold water:
2Na+2H2O→2NaOH+H2
Reaction with Acids: Metals also react with acids to produce a salt and hydrogen gas. Zinc reacts with hydrochloric acid to produce zinc chloride and hydrogen:
Zn+2HCl→ZnCl2+H2
Displacement Reactions: A more reactive metal can displace a less reactive metal from its salt solution. For instance, if you add zinc to a copper sulfate solution:
Zn+CuSO4→ZnSO4+Cu
Here, zinc displaces copper from copper sulfate due to its higher reactivity.
Various factors influence metal reactivity:
Number of Outer Electrons: Metals with only one electron in their outer shell (like potassium or sodium) tend to lose that electron more readily, making them highly reactive.
Shielding Effect: Inner shell electrons can repel outer shell electrons, making it easier for them to be lost. Metals with more inner shells generally exhibit higher reactivity.
Atomic Size: Larger atoms often lose electrons more readily due to the greater distance between the nucleus and the outermost electron.
Understanding metal reactivity is crucial for various industrial and everyday applications:
Corrosion Prevention: Knowing which metals corrode and which don’t allows us to prevent rusting and degradation, especially in structures like bridges, ships, and metallic tools.
Battery Design: Metals like lithium, which have high reactivity, are used in batteries due to their ability to release electrons quickly.
Mining and Metal Extraction: Reactive metals are often harder to extract from their ores than less reactive ones. Thus, understanding reactivity helps in choosing efficient extraction processes.
Safe Handling: Highly reactive metals need to be stored and handled under specific conditions to avoid accidents.
Predictive Abilities: A thorough grasp of metal reactivity allows scientists and industries to predict how metals will behave under specific conditions. This foresight is invaluable for research, product development, and manufacturing.
Safety Protocols: Knowing the reactivity of metals can lead to safer handling, storage, and transportation methods, especially for highly reactive metals.
Economic Benefits: By selecting metals with appropriate reactivity levels for specific applications, industries can optimise the lifespan and functionality of their products. This can lead to reduced maintenance costs and longer product life cycles.
Environmental Conservation: A deeper understanding of metal reactivity can aid in developing methods to reduce corrosion and waste, which can have significant environmental impacts. Reduced corrosion can mean a decrease in the need for replacements, leading to lower consumption of resources.
Customisation: With knowledge of reactivity, industries can tailor-make alloys (combinations of metals) to have desired properties, like resistance to rust or improved strength.
Catalysis: Many chemical reactions in industries need catalysts to speed up the process. Knowing which metals are reactive and how they react can help in selecting the best catalysts for specific reactions.
Education & Research: An understanding of metal reactivity serves as a foundational topic for students and researchers in the fields of chemistry, metallurgy, and material science, paving the way for innovations.
Cultural & Historical Understanding: The use, value, and mining of metals throughout history has often been based on their reactivity. For instance, gold’s inertness is a reason for its historical use in jewelry and currency. Understanding metal reactivity provides insights into cultural and historical practices and values.

Corrosion: Highly reactive metals tend to corrode easily. For instance, iron rusts when exposed to moisture and air, leading to degradation of structures, machinery, and tools, and incurring substantial economic costs.
Safety Concerns: Metals like sodium and potassium are so reactive that they can explode on contact with water. This poses storage, handling, and transportation challenges.
Short Lifespan of Products: Highly reactive metals, unless protected or alloyed, can have a significantly reduced lifespan, leading to the need for frequent replacements.
Environmental Concerns: The corrosion of metals can lead to the release of potentially harmful substances into the environment. For instance, when certain metals corrode, they can leach into soil or water, posing environmental hazards.
Extraction Difficulties: Highly reactive metals are often harder to extract from their ores due to their tendency to form stable compounds. This can increase the cost of extraction and the energy required, leading to a larger carbon footprint.
Alloying Challenges: To combat the challenges of high reactivity, metals are often alloyed (combined) with other elements. However, creating these alloys can be energy-intensive and can sometimes compromise other desired properties of the metal.
Economic Implications: The need to frequently replace corroded parts or structures made of reactive metals can have substantial economic repercussions, especially in industries where machinery and equipment are paramount.
Disposal Issues: Highly reactive metals can pose challenges in disposal. If not disposed of properly, they can react with environmental agents and cause pollution.
Specialised Storage Needs: Reactive metals often need specialised storage conditions, like oil immersion for alkali metals, which can increase costs and complicate logistics.
Resource Depletion: As reactive metals corrode and degrade faster, there can be an increased demand for mining and extraction, leading to faster depletion of natural resources.
A fascinating subject, metal reactivity bridges the gap between the microscopic world of atoms and electrons and the macroscopic world of industrial applications, architecture, and everyday items. Our understanding of the interactions between metals can help us better appreciate the ways in which they influence our world.
As always, thank you for checking out our blog. We hope that this helps you with your project.
Please also check out the other articles in our helpful guide series. We have written about ‘Which Metals Rust And Can It Be Prevented?‘ and ‘Benefits of Stainless Steel‘ recently so why not check them out?
We are also proud to sell this product on our highly popular eBay store, check us out there too.
If you have any further questions, feel free to contact us.





Speciality Metals
Unit 1, Farrell Street, Warrington,
Cheshire, WA1 2WW, United Kingdom
Quick Links
Payment Options
