- Massive Range
- FREE UK Delivery
- Rapid Dispatch
- Massive Range
- FREE UK Delivery
- Rapid Dispatch
- Massive Range
- FREE UK Delivery
- Rapid Dispatch
Home » Comparing the Thermal Conductivity of Different Metals

Thermal conductivity is a crucial property of materials, particularly metals, that defines their ability to conduct heat. The property of thermal conductivity is crucial for a variety of applications, ranging from heat sinks and heat exchangers to electrical components and construction materials. By understanding the thermal conductivity of different metals, one can choose the right material for a specific application while ensuring safety and efficiency.
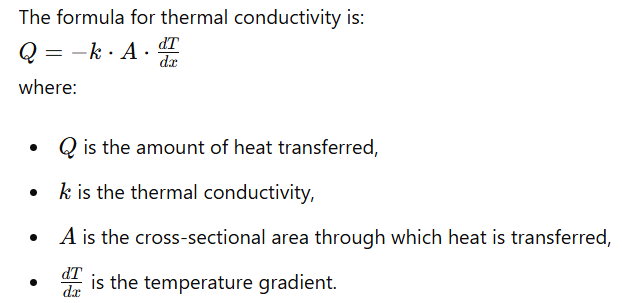
Thermal conductivity, denoted by the symbol 𝑘 or λ, is a measure of a material’s ability to conduct heat. It is defined as the quantity of heat transferred through a unit thickness of the material per unit area per unit temperature difference. The SI unit of thermal conductivity is watts per meter per kelvin (W/m·K).
Higher thermal conductivity indicates that the material is a better conductor of heat. Metals generally have high thermal conductivity compared to non-metals, making them ideal for applications requiring efficient heat transfer.
Several factors influence the thermal conductivity of metals:
Silver has the highest thermal conductivity of any metal, with a value of approximately 429 W/m·K. Its exceptional thermal properties make it ideal for high-performance thermal applications, such as in electrical contacts, thermal paste, and high-end heat sinks.
Copper is well-known for its excellent thermal conductivity, around 401 W/m·K. It is widely used in electrical wiring, heat exchangers, and cooking utensils. Copper’s thermal performance, combined with its electrical conductivity, makes it indispensable in many industries.
Gold has a thermal conductivity of about 318 W/m·K. Although it is less commonly used for its thermal properties due to its high cost, gold is utilized in specific high-reliability applications, such as in electronics and thermal management in aerospace technology.
Aluminum has a thermal conductivity of approximately 237 W/m·K. It is lightweight and has good corrosion resistance, making it a popular choice for applications like automotive radiators, air conditioning units, and heat sinks for electronic devices.
Iron has a relatively lower thermal conductivity of around 80 W/m·K. It is commonly used in construction and automotive industries where high thermal conductivity is not critical.
Steel, an alloy primarily composed of iron and carbon, has a thermal conductivity that varies with its composition. On average, carbon steel has a thermal conductivity of about 45-58 W/m·K, while stainless steel ranges from 15-30 W/m·K. Steel is widely used in construction, automotive, and industrial applications.
Brass, an alloy of copper and zinc, has a thermal conductivity of about 109 W/m·K. It is used in applications where a combination of good thermal conductivity and corrosion resistance is needed, such as in heat exchangers, plumbing, and musical instruments.
Titanium has a relatively low thermal conductivity of about 22 W/m·K. It is highly valued for its strength, corrosion resistance, and low density, making it suitable for aerospace, medical implants, and marine applications.
Nickel has a thermal conductivity of approximately 90 W/m·K. It is used in applications like batteries, catalysts, and special alloys due to its chemical stability and resistance to corrosion.
Lead has a thermal conductivity of around 35 W/m·K. It is used in applications requiring radiation shielding, soundproofing, and in some types of batteries.

Materials with high thermal conductivity, such as copper and aluminum, are essential for manufacturing heat sinks and heat exchangers. These devices dissipate heat from electronic components, engines, and industrial processes to prevent overheating and ensure efficient operation.
Metals like silver and copper, with both high electrical and thermal conductivity, are used extensively in electrical components, including wiring, connectors, and circuit boards. Efficient heat dissipation is crucial in preventing electrical failures and improving the longevity of components.
In construction, materials with lower thermal conductivity, such as steel, are used where thermal insulation is beneficial. For example, steel’s moderate thermal conductivity makes it suitable for structural applications without significant heat loss or gain.
Copper and aluminum are popular choices for cooking utensils due to their high thermal conductivity, which ensures even heat distribution. This property helps in cooking food uniformly and efficiently.
In aerospace and automotive industries, materials like titanium and aluminum are used for their combination of thermal conductivity, strength, and lightweight properties. These metals are crucial in applications where weight reduction and heat management are critical, such as in aircraft frames and engine components.
Titanium’s low thermal conductivity, along with its biocompatibility and corrosion resistance, makes it an ideal material for medical implants. The lower thermal conductivity helps in maintaining body temperature stability around the implant.
Recent advancements in nanotechnology have led to the development of materials with tailored thermal properties. For example, graphene and carbon nanotubes exhibit exceptionally high thermal conductivity and are being explored for advanced thermal management applications.
Composite materials, combining metals with ceramics or polymers, can achieve a balance of thermal conductivity, mechanical strength, and weight. These materials are being developed for use in aerospace, automotive, and electronics industries.
Phase change materials (PCMs) are being integrated with metals to enhance thermal storage and management. These materials absorb and release heat during phase transitions, providing effective thermal regulation in applications like thermal energy storage and temperature control in electronic devices.
| Metal | Thermal Conductivity (W/m·K) | Pros | Cons |
|---|---|---|---|
| Silver | 429 | Highest thermal conductivity, excellent for high-performance applications | Expensive, limited use due to cost |
| Copper | 401 | High thermal conductivity, widely available, good electrical conductivity | Susceptible to oxidation, relatively heavy |
| Gold | 318 | High thermal conductivity, excellent corrosion resistance, good electrical conductivity | Very expensive, limited use to specific applications |
| Aluminum | 237 | High thermal conductivity, lightweight, corrosion-resistant | Lower thermal conductivity than silver and copper, softer metal |
| Iron | 80 | Abundant, inexpensive, useful in structural applications | Lower thermal conductivity, prone to rust |
| Steel | 45-58 (Carbon Steel), 15-30 (Stainless Steel) | Strong, versatile, used in many structural applications | Lower thermal conductivity, can corrode unless stainless |
| Brass | 109 | Good thermal conductivity, corrosion-resistant, aesthetically pleasing | Heavier than aluminum, lower conductivity than pure copper |
| Titanium | 22 | Lightweight, strong, corrosion-resistant, biocompatible | Low thermal conductivity, expensive |
| Nickel | 90 | Good thermal conductivity, excellent corrosion resistance | Expensive, heavier than aluminum and brass |
| Lead | 35 | Dense, good radiation shielding, soundproofing | Toxic, very low thermal conductivity, heavy |
The thermal conductivity of different metals varies significantly, influenced by factors such as atomic structure, temperature, impurities, and microstructure. The right material must be selected for specific applications based on these variations in order to ensure optimal performance and efficiency.
The use of metals with high thermal conductivity is essential for applications such as heat sinks, electrical components, and kitchen utensils, which rely on efficient heat transfer. Steel and titanium, on the other hand, have lower thermal conductivity, making them excellent for applications in construction and aerospace.
Technology continues to develop new materials and composites with tailored thermal properties, enhancing performance across several industries. A wide range of applications will continue to benefit from understanding and harnessing the thermal conductivity of different metals.
Metal’s thermal conductivity greatly influences their applications across industries as a fundamental property. Designing products and systems based on the thermal properties of metals will enable engineers and designers to progress technologically and improve the quality of life.
As always, thank you for checking out our blog. We hope that this helps you with your project.
Please also check out the other articles in our helpful guide series. We have written about Ferrous vs. Non-Ferrous Metals and Comparing Aluminium, Mild Steel, and Stainless Steel recently to name but two of our articles.
We are also proud to sell this product on our highly popular eBay store, check us out there too.
If you have any further questions, feel free to contact us.













Speciality Metals
Unit 1, Farrell Street, Warrington,
Cheshire, WA1 2WW, United Kingdom
Quick Links
Payment Options
